Finite Element Modelling Of Thermal Processes With Phase=
Transitions
Abdelhaq Abouhafc
Site of the project:
TNO Science and Industry
Schoemakerstraat 97
2628 VK
Delft
start of the project: September 2006
In December 2006 the
Interim
Thesis has been appeared
and a
presentation has been given.
The Master project has been finished in June 2007 by the completion of
the Masters Thesis and a final
presentation has been given.
For working address etc. we refer to our alumnipage.
Summary of the master project:
Adding and removing heat and mass to and from a product take place in
several
processes in the industry. For example: steam production, freezing and
drying.
Improving product quality is the objective of using physical and
chemical models. These models solve a set of heat and mass equations as a function of
time
and position. The pressure is assumed to be constant, while the
temperature
and concentration are chosen as state variables. When phase change takes
place
during these processes the modeling becomes more complicated. In this
case
using only temperature and concentration is not sufficient for the
process description. Additional state variables such as solid, liquid and vapor
fractions
are needed.
These processes are encountered by oil refineries

The simulation model should decide which equations are appropriate
at a
given time during the simulation; one should also take into account that
the
phase changes usually occur during a part of the entire process. The
simulation
of thermal process with phase changes is usually quite difficult because
we need
to solve different sets of equations for different phases of the
process. Switching
from one set of equations to another can cause instability of the
numerical solution. We also need to use a large amount of input parameters, such as
specific
heat and densities of all phases and latent heat of phase changes.
The objective of this project is the combination of the
density-enthalpy phase
diagram with finite elements methods. The finite element method can be
used
to solve transport equations (mass, heat and impulse) and the
density-enthalpy
phase diagram gives the thermodynamics constants. The permeability,
viscosity
and heat conductance are very important in this case. The developed
models
can be used to optimize processes and products quality.
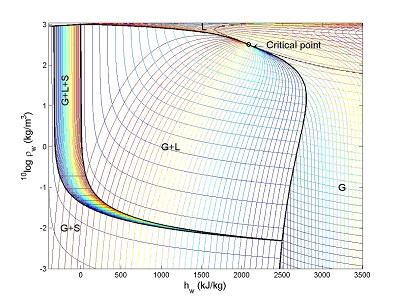
The density-enthalpy phase
diagram for water

Contact information:
Kees
Vuik

Back to the
home page
or the
Master students page of Kees Vuik


![]()
![]()